Recently, a research team led by Professor Li Chunju from the School of Chemistry at Tianjin Normal University (TNU) has developed a simple, efficient, and versatile synthetic strategy—termed Intramolecular Coupling of Extended Biphen[n]arenes (ICEB)—for the precise synthesis of cycloparaphenylene derivatives. This breakthrough enables the tailored construction of a series of conjugated nanoring molecules with customizable sizes and skeletal motifs. The related findings have been published in Nature Synthesis under the title "Customized Cycloparaphenylene Skeletons Prepared via the Intramolecular Coupling of Extended Biphen[n]arenes." Nature Synthesis is an internationally authoritative journal under the Nature portfolio focusing on chemical synthesis. Dr. Du Xusheng is the first author of the paper, with Professor Li Chunju serving as the corresponding author. Co-authors include Professor Cai Kang and Professor Guo Dongsheng from Nankai University, and Professor Jonathan L. Sessler from The University of Texas at Austin.
Cycloparaphenylenes (CPPs) are a class of conjugated nanoring molecules with unique electronic structures and photophysical properties. Regarded as the smallest repeating units of carbon nanotubes, CPPs have attracted widespread attention since their first synthesis in 2008. Their distinctive radially conjugated π-electron systems and nanoscale rigid cavities not only provide an ideal model for exploring the effects of dimensional changes on the electronic properties of carbon materials but also point toward new directions for developing novel organic optoelectronic materials, molecular containers, and bio-probes. Notably, CPPs are considered pivotal "seeds" or templates for the precise chemical synthesis of carbon nanotubes, holding promise to fundamentally address the bottlenecks of uncontrollable chirality and size in traditional preparation methods, thereby advancing nanocarbon materials toward on-demand "molecular manufacturing." However, the synthesis of CPPs themselves is hindered by high ring strain, tedious steps, and generally low yields. A deeper understanding and precise tuning of their electronic properties remain insufficient. More crucially, how to use CPPs as building blocks for the directional and efficient extension into long-range ordered carbon nanotube structures through controlled chemical reactions remains a core scientific challenge. Therefore, research on the synthetic methodologies, property modulation, and functional applications of CPP systems is essential for breaking through current limitations and realizing their theoretical value and practical potential.
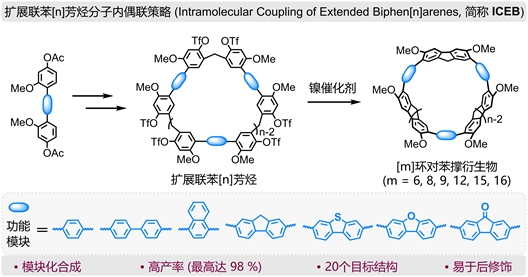
Current mainstream synthetic strategies primarily include two approaches: the reduction or oxidative aromatization of non-conjugated macrocyclic precursors, and the metal-mediated reductive cyclization of organometallic macrocyclic precursors. In recent years, using macrocyclic arenes as precursors to synthesize strained nanorings has gradually emerged as a new research direction. Building on previous work, Professor Li Chunju's team, in collaboration with Professors Cai Kang and Guo Dongsheng from Nankai University and Professor Jonathan L. Sessler from the University of Texas at Austin, has developed a new versatile synthetic strategy—the Intramolecular Coupling of Extended Biphen[n]arenes (ICEB). This strategy enables systematic control over the ring size, molecular structure, substituent type, and photophysical properties of CPP derivatives. The core of this strategy lies in employing extended biphen[n]arenes containing multiple aromatic repeating units as precursors. Their larger ring size significantly reduces the number of chemical bonds required during cyclization, effectively lowering the ring strain in the product and thereby greatly enhancing the efficiency and yield of the intramolecular coupling reaction. Simultaneously, these precursors feature highly modular and customizable properties, allowing for flexible adjustment of aromatic unit types and connection modes, as well as precise introduction of specific functional groups. This facilitates subsequent chemical modifications, significantly enhances the structural diversity and functional tunability of the products, and further broadens their application prospects in supramolecular chemistry and advanced functional materials.
Based on this simple, efficient, and broadly applicable synthetic strategy, the research team successfully constructed 20 structurally diverse CPP derivatives. Analyses using UV-Vis absorption spectroscopy, fluorescence spectroscopy, and density functional theory calculations indicate that the absorption and emission wavelengths of these molecules can be precisely tuned over a wide range by altering the electronic characteristics of the embedded aromatic units and the ring size. The ICEB strategy offers a simple synthetic method with high yield, demonstrating good versatility and operability. It serves as a powerful complement to existing conjugated nanoring synthesis systems and will provide new insights for the rational design and controlled synthesis of complex strained carbon architectures, such as carbon nanocages, carbon nanotube segments, and mechanically interlocked molecules.
Article links: https://www.nature.com/articles/s44160-025-00965-7
By He Jierui