Professor Wang Chao from the School of Chemistry at Tianjin Normal University (TNU) has made progress in the study ofiron-catalyzed asymmetric reductive coupling. The related findings, titled "Iron-Catalyzed Asymmetric Reductive Cross-Coupling of Ketimines with Alkyl Iodides", were published online in the "Journal of the American Chemical Society".
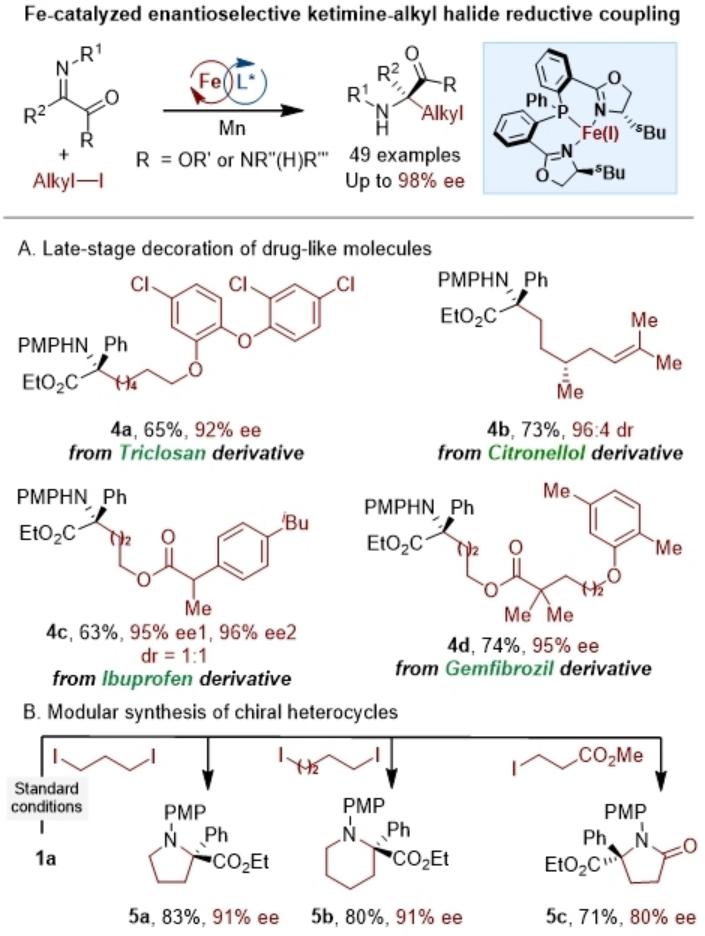
Figure. Iron-Catalyzed Asymmetric Reductive Cross-Coupling of Imines with Alkyl Iodides and Pharmaceutical Modification
Transition metal-catalyzed asymmetric cross-coupling reactions have revolutionized the stereoselective construction of chiral structures and are widely applied in the synthesis of pharmaceuticals, agrochemicals, and functional materials. Early research predominantly focused on second-row transition metal systems such as ruthenium, rhodium, and palladium. However, in recent years, first-row transition metals (e.g., nickel, copper, cobalt) have gained increasing attention due to their advantages of low cost, abundance, and low toxicity. Among them, iron, owing to its high crustal abundance and minimal biological toxicity, is considered one of the most promising metals for sustainable catalysis. Nevertheless, the development of iron-catalyzed asymmetric cross-coupling has lagged, primarily due to its complex oxidation states, spin states, and multiple reaction pathways, which make organoiron intermediates difficult to characterize and pose challenges in designing chiral ligands that effectively control reactivity and stereoselectivity.
Addressing these challenges, Professor Wang Chao's research group developed an iron-catalyzed asymmetric reductive cross-coupling strategy between ketimines and alkyl iodides, enabling the efficient construction of α-tertiary amino esters and amides. This method employs a combination of commercially available iron (II) triflate and a chiral bis(oxazoline)-phosphine (NPN) ligand, with inexpensive manganese powder as the reducing agent. A variety of primary and secondary alkyl iodide substrates were converted into target products in high yields and with excellent enantioselectivity, demonstrating remarkable functional group tolerance. This strategy is not only suitable for late-stage modification of complex molecules but can also construct heterocyclic compounds containing quaternary stereocenters. Mechanistic studies, including radical clock experiments and high-resolution mass spectrometry, indicate that the reaction follows an Fe(I)/Fe(II)/Fe(III) redox cycle and involves a single-electron transfer (SET) process with alkyl radical intermediates. This work not only expands new pathways for asymmetric C(sp³)–C bond formation but also demonstrates the significant potential of chiral iron catalysis in stereoselective radical reactions.
Professor Wang Chao is the corresponding author of the paper, and graduate students Wang Jiaxin and Bai Haohao are co-first authors. TNU is the corresponding affiliation. This research was supported by the National Natural Science Foundation of China (21901185), the Tianjin Natural Science Foundation (23JCYBJC00760), and the Startup Fund for High-Level Talents at TNU.
Article link: https://pubs.acs.org/doi/10.1021/jacs.5c11470
By He Jierui